Variable importance in survival analysis: multi-seed estimation
Source:vignettes/v3_sample_splitting.Rmd
v3_sample_splitting.Rmd
library(survML)
#> Loading required package: SuperLearner
#> Loading required package: nnls
#> Loading required package: gam
#> Loading required package: splines
#> Loading required package: foreach
#> Loaded gam 1.22-6
#> Super Learner
#> Version: 2.0-29
#> Package created on 2024-02-06
library(survival)
library(dplyr)
#>
#> Attaching package: 'dplyr'
#> The following objects are masked from 'package:stats':
#>
#> filter, lag
#> The following objects are masked from 'package:base':
#>
#> intersect, setdiff, setequal, union
set.seed(72924)Introduction
As discussed in the variable importance overview vignette, in order to obtain valid inference for VIM under the null hypothesis of zero importance, we employ sample-splitting. We also use cross-fitting in order to allow the use of flexible machine learning algorithms for nuisance parameter estimation. (For a general overview of variable importance, read the overview vignette first. We use the same notation here.)
We recall that the importance of a covariate set relative to a larger covariate set is ; the difference in maximum achievable predictiveness when only is excluded compared to when is excluded from the full covariate set . Sample-splitting entails estimating and using separate portions of the data. This serves a distinct purpose from cross-fitting, which involves dividing the data into folds, estimating the nuisance parameters using folds, estimating predictiveness using the remaining holdout fold, repeating this process for all folds, and averaging the results. We recommend using cross-fitting in conjunction with sample-splitting, as illustrated in the figure below.
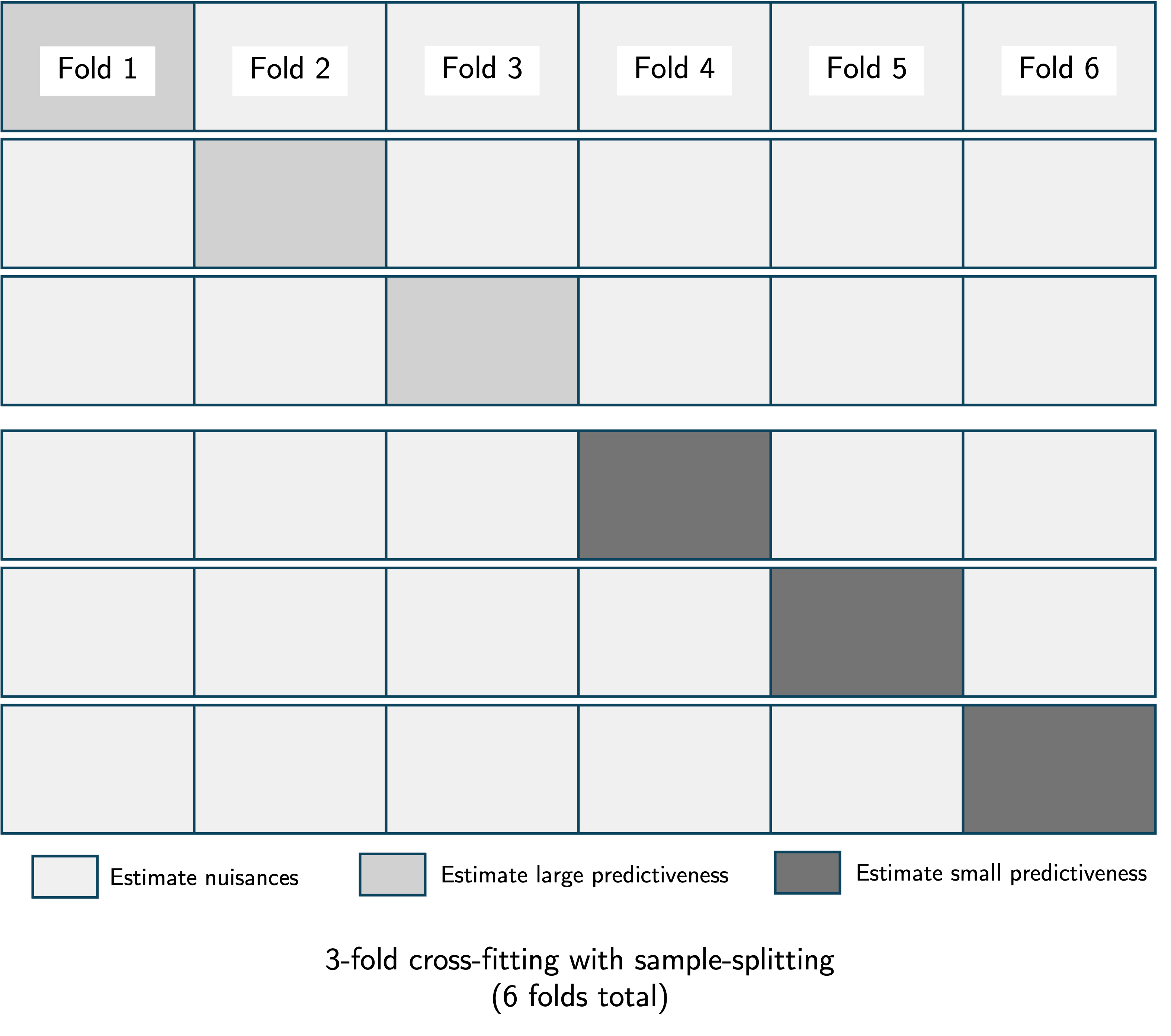
While cross-fitting does not change the large-sample variance of the VIM estimator, sample-splitting does lead to an estimator with larger variance. Furthermore, both sample-splitting and cross-fitting introduce additional randomness into the inferential procedure due to the random allocation of data units into folds.
Muli-seed VIM estimation
Each iteration of the VIM procedure depends on the seed selected by the user. The seed determines the pseudo-random process by which the data are divided into folds, along with other stochastic components of the VIM estimation procedure (e.g., cross-validation for selection of algorithm tuning parameters).
In order to mitigate this randomness, we generally recommend
performing the VIM procedure multiple times using different seeds, and
then aggregating the results. This functionality is carried out by the
multiseed_vim() function. Suppose we use
different seeds to perform the VIM analyses. We denote the VIM point
estimate produced by the
th
seed as
,
and the corresponding estimated (scaled) variance as
.
Aggregating the point estimates and inferential results across the
iterations require different approaches.
Point estimates
We have point estimates . Because all of these point estimates are consistent for the true VIM , many aggregation strategies will produce valid results. We recommend simple averaging, constructing an overall VIM estimate .
Inference
Combining the inferential results — confidence intervals and -values — across multiple seeds is more complicated. Each of the iterations of the procedure produces a valid hypothesis test of the null hypothesis for any value (most often, we are interested in testing — that is, zero importance — but we can test any point null). Thus, aggregating the -values from the various seeds in a manner that controls the Type I error of the aggregated hypothesis test allows us to obtain a single -value corresponding to a hypothesis test of .
There is a large literature on combining multiple -values for testing the same hypothesis; see Vovk and Wang (2020) for an in-depth analysis of potential aggregation methods. Example aggregation methods include Bonferroni (multiply the smallest -value by the number of seeds) and the arithmetic mean (take the arithmetic mean of the -values and multiply by two).
In survML, we use the Wald test statistic
to test
.
We denote the resulting
-value
as
.
We apply some aggregation function
to produce a combined
-value
.
A
confidence interval for
consists of all values
for which
,
i.e., for which we do not reject the null
.
If the
-values
correspond to a one-sided test, the resulting interval is one-sided
(i.e., it is of the form
for a lower bound
);
if the
-values
correspond to a two-sided test, the resulting interval is two-sided. The
multiseed_vim() function produces both types of intervals,
along with the aggregated
-value
corresponding to a one-sided test of
(zero importance).
Example: Predicting recurrence-free survival time in cancer patients
As in the variable importance overview
vignette, we consider the importance of various features for
predicting recurrence-free survival time using the gbsg
dataset from the survival package. For illustration, we
look at the importance of tumor-level features (size, nodes, estrogen
receptor, progesterone receptor, and grade) relative to the full feature
vector.
There are three arguments unique to multiseed_vim()
compared to the standard vim() function. The first is
n_seed, which determines the number of iterations to
perform. The second is ci_grid, which determines the values
of
for which hypothesis tests are performed. This argument should
correspond to a range of values over which a feasible confidence
interval could span. For example, for AUC predictiveness, the importance
of a feature cannot be outside of
,
so these are reasonable bounds for ci_grid. The third
argument is agg_method, which determines the
-value
aggregation method. Vovk and Wang (2020) found the compound
Bonferroni-geometric mean method "compound_bg" to work well
in simulations; this is the default for multiseed_vim().
Below, we compare the results of a single call to vim()
versus the multiseed approach with 3 seeds.
data(cancer)
### variables of interest
# rfstime - recurrence-free survival
# status - censoring indicator
# hormon - hormonal therapy treatment indicator
# age - in years
# meno - 1 = premenopause, 2 = post
# size - tumor size in mm
# grade - factor 1,2,3
# nodes - number of positive nodes
# pgr - progesterone receptor in fmol
# er - estrogen receptor in fmol
# create dummy variables and clean data
gbsg$tumgrad2 <- ifelse(gbsg$grade == 2, 1, 0)
gbsg$tumgrad3 <- ifelse(gbsg$grade == 3, 1, 0)
gbsg <- gbsg %>% na.omit() %>% select(-c(pid, grade))
time <- gbsg$rfstime
event <- gbsg$status
X <- gbsg %>% select(-c(rfstime, status)) # remove outcome
# find column indices of features/feature groups
X_names <- names(X)
tum_index <- which(X_names %in% c("size", "nodes", "pgr", "er", "tumgrad2", "tumgrad3"))
landmark_times <- c(1000, 2000)
output_single <- vim(type = "AUC",
time = time,
event = event,
X = X,
landmark_times = landmark_times,
large_feature_vector = 1:ncol(X),
small_feature_vector = (1:ncol(X))[-as.numeric(tum_index)],
conditional_surv_generator_control = list(SL.library = c("SL.mean", "SL.glm"),
V = 2,
bin_size = 0.5),
large_oracle_generator_control = list(SL.library = c("SL.mean", "SL.glm"),
V = 2),
small_oracle_generator_control = list(SL.library = c("SL.mean", "SL.glm"),
V = 2),
approx_times = sort(unique(stats::quantile(time[event == 1 & time <= max(landmark_times)],
probs = seq(0, 1, by = 0.025)))),
cf_fold_num = 2,
sample_split = TRUE,
scale_est = TRUE)
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
output_single$result
#> landmark_time est var_est cil ciu cil_1sided
#> 1 1000 0.18408503 0.5787338 0.1035768 0.2645933 0.1165204
#> 2 2000 0.05050178 2.6346037 0.0000000 0.2222763 0.0000000
#> p large_predictiveness small_predictiveness vim
#> 1 3.705528e-06 0.7049526 0.5208675 AUC
#> 2 2.822298e-01 0.6523270 0.6018252 AUC
#> large_feature_vector small_feature_vector
#> 1 1,2,3,4,5,6,7,8,9 1,2,7
#> 2 1,2,3,4,5,6,7,8,9 1,2,7
output_multiseed <- multiseed_vim(n_seed = 3,
ci_grid = seq(0, 1, by = 0.01),
type = "AUC",
agg_method = "compound_bg",
time = time,
event = event,
X = X,
landmark_times = landmark_times,
large_feature_vector = 1:ncol(X),
small_feature_vector = (1:ncol(X))[-as.numeric(tum_index)],
conditional_surv_generator_control = list(SL.library = c("SL.mean", "SL.glm"),
V = 2,
bin_size = 0.5),
large_oracle_generator_control = list(SL.library = c("SL.mean", "SL.glm"),
V = 2),
small_oracle_generator_control = list(SL.library = c("SL.mean", "SL.glm"),
V = 2),
approx_times = sort(unique(stats::quantile(time[event == 1 & time <= max(landmark_times)],
probs = seq(0, 1, by = 0.025)))),
cf_fold_num = 2,
sample_split = TRUE,
scale_est = TRUE)
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
#> Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
output_multiseed$agg_result
#> landmark_time est var_est cil ciu cil_1sided p
#> 1 1000 0.1718534 0.5610887 0.09 0.27 0.10 7.046226e-06
#> 2 2000 0.1746481 2.9069883 0.00 0.32 0.02 2.665718e-02
#> large_predictiveness small_predictiveness vim large_feature_vector
#> 1 0.7079477 0.5360943 AUC 1,2,3,4,5,6,7,8,9
#> 2 0.7174815 0.5428334 AUC 1,2,3,4,5,6,7,8,9
#> small_feature_vector
#> 1 1,2,7
#> 2 1,2,7References
The survival variable importance methodology is described in
Charles J. Wolock, Peter B. Gilbert, Noah Simon and Marco Carone. “Assessing variable importance in survival analysis using machine learning.” Biometrika (2025).
Methods for aggregating -values are discussed in
Vladimir Vovk and Ruodu Wang. “Combining p-values via averaging.” Biometrika (2020).